Literature Quick Reading | Long-term Culture, Genetic Manipulation, and Xenotransplantation of Human Normal and Breast Cancer Organoids
Organoid technology has transformed the study of human organ development, disease, and treatment response for individuals. Although detailed protocols are available for the generation and long-term propagation of human organoids from a variety of organs, such methods are lacking for breast tissue. Here we provide an optimized and highly versatile protocol for the long-term culture of organoids from normal human breast tissue or breast cancer (BC) tissue. In addition, we provide methods for genetic manipulation by Lipofectamine 2000, electroporation, or lentivirus and subsequent selection and clonal culture of organoids. Organoids derived from tissue fragments require 7-21 days to reach the first division; the generation of genetically manipulated clonal organoid cultures requires 14-21 days; and the expansion of organoids for xenotransplantation requires >4 weeks.
Experimental Design
PART1 Expansion Medium (Reagent Preparation)
We first describe the preparation of a previously published expansion medium (referred to as "Type 1"; Table 1). In addition, we describe another expansion medium, similar to the expansion medium developed for long-term ovarian cancer organoid cultures, referred to as "Type 2"; Table 1), which can improve the growth characteristics of some organoid cultures (Figure 2a). Images of growth in Type 1 or Type 2 medium show that Type 2 organoids grow faster compared to Type 1. We recommend culturing newly established organoid cultures in both Type 1 and Type 2 expansion medium to determine the optimal medium type for each donor. Here we used homemade R-spondin-1, Noggin, and Wnt3a conditioned medium.
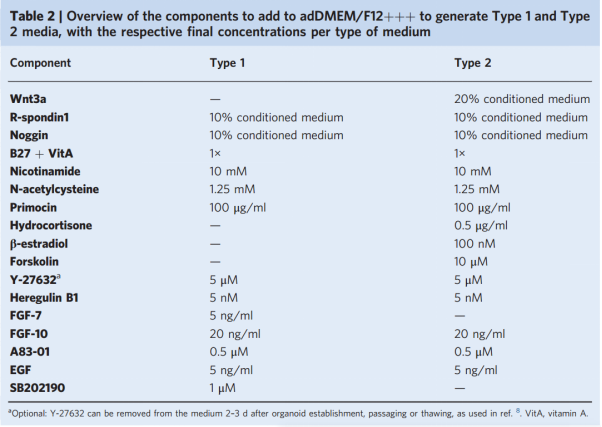
Table 1 Components added to adDMEM/f12++ to generate type 1 and type 2 media, and the final concentration of each medium

Figure 1. Derivation, culture and passaging of organoids
PART2 Organoid culture
After the organoid culture system is established, the patient's resection material is cut into small pieces, thoroughly washed and digested with collagenase. After washing and filtering, the digested tissue is placed in basement membrane extract (BME) and supplemented with expansion medium (Figure 1b, c). BME is a gel-like substance that polymerizes at temperatures above 10°C and is used to mimic the extracellular matrix and provide support for 3D organoids (Figure 1d, e) Figure d is a representative image of organoids after washing: no particles (left), particles containing residual BME (middle) and clean particles (right). Basement membrane matrix can be used as a substitute for BME with similar properties (Figure 1f). Organoids are best used for tissues containing small amounts of fat and necrotic tissue. The epithelial tissue required for the generation of organoids usually appears as hard, pink to brown tissue, while necrotic tissue is usually softer and darker in color. Adipose tissue is soft, white to yellow in color, and can be easily scraped or cut away.
PART3 Organoid Maintenance
Organoids can be passaged 7-21 days after their establishment or at a ratio of 1:2-1:8 after the last split. The passaging time and split ratio of newly established organoid cultures should be optimized based on confluence. The optimal passaging time is before the organoids in the center of the BME droplet begin to die (manifested by the shedding of debris or darkening of color) or when the organoid diameter is greater than 300μm (further referred to as "confluent wells"). We describe both single-cell organoid passaging by trypsinization (Figure 1g) and organoid fragment passaging by mechanical disruption (called shearing) (Figure 1g).
We also provide guidelines for culturing at appropriate organoid density and describe how to minimize cell death and identify confluent organoids (Figure 1h-j). In addition, we describe procedures and conditions for long-term storage and recovery after cryopreservation. We recommend freezing at least 6 cryovials at early stages (<5 stages) for each newly established organoid culture.
PART4 Genetic Manipulation
We discuss three different methods for genetic modification of mammary organoids, including Lipofectamine 2000 for medium- and high-efficiency transient transfection, electroporation for high-efficiency transient transfection, and lentiviral infection for stable DNA transduction (Figure 2a-c). Specific conditions (e.g., DNA concentration, viral titer, and organoid cell number) or settings (e.g., voltage used for electroporation) need to be optimized for each experiment and culture. We recommend using control transfections or transduction vectors with fluorescent markers to assess the efficiency of the procedure (Figure 2b-e).
PART5 Organoid Selection and Clonal Culture
If resistance genes have been introduced, transgenic organoids can be selected by adding antibiotics or by adding or removing other media components. For example, adding Nutlin-3a will kill all cells expressing wild-type P53 and can be used to select organoids with P53 mutations (Figure 2c). In addition, discontinuing epidermal growth factor (EGF) can select cells that overexpress KRAS23. We recommend determining the optimal concentration for each selection agent and organoid culture, which is generally the lowest concentration that kills 100% of untreated organoids. Selection and propagation of single organoid tissue can be used to generate clonal cultures (Figure 2d, e). Untreated organoids should be used as controls. Although subclones of each organoid culture can be evaluated, we recommend using organoid cultures that can be efficiently expanded after single-cell passaging to increase the success rate.
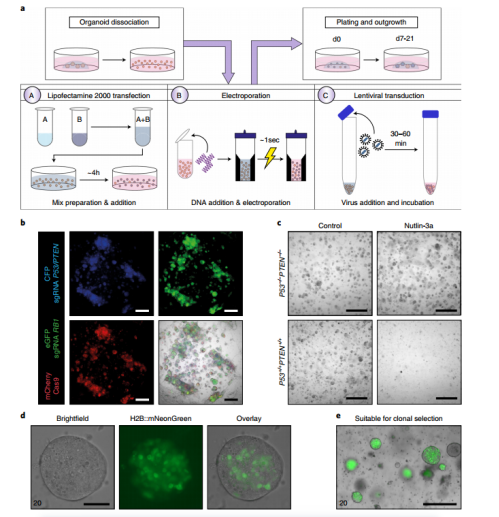
Figure 2 Genetic manipulation of mammary organoids a. Schematic diagram of genetic manipulation of mammary organoids. Dissociated organoids can be incubated with a Lipofectamine 2000-based transfection mix (A), electroporated (B), or incubated with high-titer lentivirus (C) and plated in BME. b. Representative fluorescent images of normal mammary organoids cultured for 7 days after transduction with lentivirus expressing fluorescent reporter genes and single guide RNA or Cas9 c. Representative bright field images of normal mammary organoids (control) or normal mammary organoids knocked out of P53 and PTEN by CRISPR-Cas9 and treated with Nutlin-3a (10 μM) for 7 days. d,e. Representative images of single organoids (d) or multiple organoids (e) that were gene-edited to stably express H2B::mNeonGreen and puromycin resistance genes after 14 days of puromycin selection.
PART6 Xenotransplantation
Transplantation requires a large number of organoids, usually 0.25×106 to 1×106 whole organoid cells per injection site, to obtain better engraftment rates than single cells.
Expected Results
Organoids derived from BC tissue can exhibit solid morphology (~60% of cultures), cystic morphology (<5%), grape-like morphology (~20%), or mixed morphology (~20%) (Fig. 3a,b). The growth rate of BC organoids varies greatly, but high expansion rates can be achieved in some donors (1:6 per week). Once established (e.g., >5 generations in vitro), BC organoids rarely stop growing or reduce their growth rate. Organoid establishment efficiency ranges from 55% to 70% for most BC subtypes and is approximately 40% for triple-negative breast cancer (TNBC; Fig. 3c,d). This suggests that TNBC cells, which are often aggressive and genetically unstable, are very challenging to adapt to in vitro culture conditions. However, since these data were collected while we were optimizing our method, the success rate may be greatly improved if Table 1 is used as a reference.
Immunohistochemical comparison with the original tumor specimens showed that organoids sometimes lost ER (~25% of samples), PR (~25% of samples), or HER2 (~20% of samples) in culture, and rarely gained ER (~10%) or HER2 (<5%) expression (Figure 3e, f). The timing of receptor expression reduction or increase during culture may vary and should be evaluated for each culture. The reasons for these changes are currently unknown but may be influenced by factors such as propagation of organoids in type 1 or type 2 expansion media, culture-induced gene silencing, or growth of certain subclones. Of note, organoids generated after tumor resection may be contaminated with normal cells, which is expected to occur in about 10% of tumor samples. However, because normal organoids generally grow slowly and eventually stop growing, it is likely that normal cells are lost in culture over time. When tumors carry p53 mutations, Nutlin-3a can be added to the expansion medium to select for tumor cells, ensuring the death of all normal cells that may be present (Figure 2c). Using BC organoids, we illustrate the potential of targeting BCL2 in ER+ BC organoids in vitro in combination with CDK4/6 inhibitors and further demonstrate that BC cultures can retain responsiveness or resistance to HER2 blockade with afatinib in vitro.
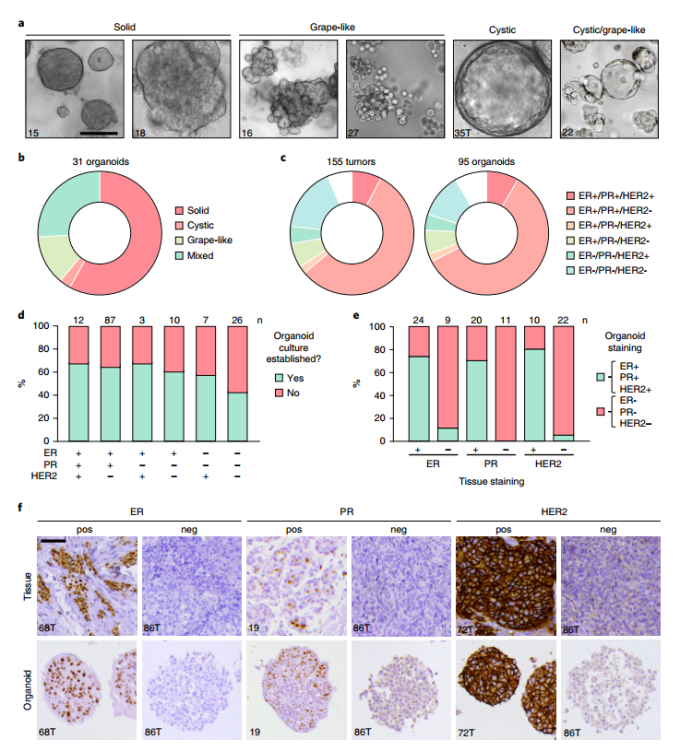
Figure 3. BC organoid culture traits a, Representative images of different morphologies of BC organoids. b, Donut plot depicting the main morphological distribution of 31 BC organoid cultures. c, Distribution of BC subtypes in 155 tissue samples and 95 related organoid cultures. d, Stacked bar graph showing the success rate of organoid establishment, grouped by receptor expression status. ‘Yes’ indicates successful establishment (> 5 in vitro passages); ‘No’ indicates unsuccessful establishment. e, Stacked bar graph showing the percentage of organoid cultures with positive (green) or negative (pink) receptor status compared to the original tissue receptor status. f, Comparative immunohistochemistry images of BC tissue and derived organoids, representative images of receptor positive (pos) and receptor negative (neg) samples.
We provide different options to alter the proportions of basal cells, luminal progenitors, and mature luminal cells. These manipulations can be used for short-term studies of specific cell types but will affect morphology and long-term growth in culture. In addition, we performed gene editing of normal breast organoids using CRISPR-Cas9 and showed that P53, PTEN, and RB1 mutations in normal organoids are sufficient to convert them into organoids that form ER+ tumors following in vivo xenografting. This illustrates the potential utility of this model in enhancing the understanding of early molecular programs that ultimately form specific subtypes of BC.
For more experimental schemes and steps of the article, please refer to the literaturehttps://doi.org/10.1038/s41596-020-00474-1
News
-
 NewsHonor | East-Mab Bio Biotech won the title of "Excellent Partner of Biochemical and Biological Enterprises"2023.12.29
NewsHonor | East-Mab Bio Biotech won the title of "Excellent Partner of Biochemical and Biological Enterprises"2023.12.29 -
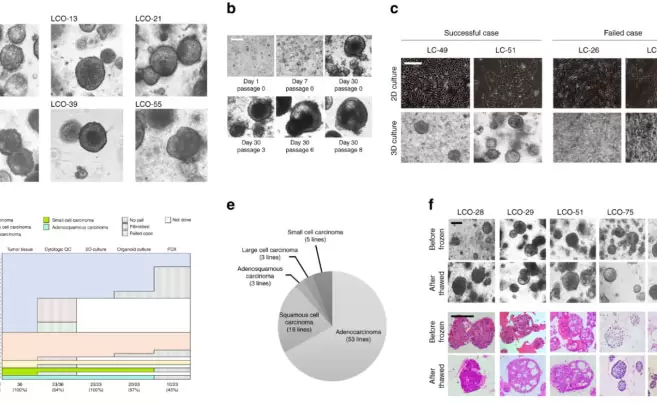 NewsQuick Reading | Patient-derived Lung Cancer Organoids as In Vitro Cancer Models for Therapeutic Screening2024.01.05
NewsQuick Reading | Patient-derived Lung Cancer Organoids as In Vitro Cancer Models for Therapeutic Screening2024.01.05 -
 NewsGood news | East-Mab Bio Biotech was recognized as "Nantong Municipal Enterprise Technology Center"2024.02.22
NewsGood news | East-Mab Bio Biotech was recognized as "Nantong Municipal Enterprise Technology Center"2024.02.22



