Quick Reading | Patient-derived Lung Cancer Organoids as In Vitro Cancer Models for Therapeutic Screening
Abstract:
Lung cancer is highly genetically and phenotypic, and personalized medicine is urgently needed. Here we report the establishment of lung cancer organoids and normal bronchial organoids from patient tissues, including five histological subtypes of lung cancer and non-neoplastic bronchial mucosa, as in vitro models representing individual patients. Lung cancer organoids recapitulate the tissue architecture of primary lung tumors and maintain the genomic alterations of the original tumor during long-term expansion in vitro. Normal bronchial organoids maintain the cellular composition of normal bronchial mucosa. Lung cancer organoid tissues respond to drugs according to their genomic alterations: BRCA2 mutant organoid tissues respond to olaparib, epidermal growth factor receptor mutant organoid tissues respond to erlotinib, and epidermal growth factor receptor mutant/MET amplification organoid tissues respond to crizotinib. Given the short time from organoid establishment to drug trials, our newly developed model may prove useful in predicting patient-specific drug responses through in vitro patient-specific drug trials.
Introduction
Organoid models of tracheobronchial and alveolar tissues are derived from adult stem cells or pluripotent stem cells and recapitulate the epithelial tissues of these two different tissue types in vitro. As personalized models of lung cancer, in vitro tissue culture or tumor spheroid culture using three-dimensional culture systems have been studied to predict the response to anticancer therapies, but their growth or recapitulation of the original tumor architecture is limited. Recently, studies have been reported to culture lung cancer organoids (LCOs) using airway organoid culture systems. However, this approach has some limitations in terms of lung cancer-specific growth and representation of the diversity of lung cancer. This paper summarizes the organoids derived from primary lung cancer tissue (LC tissue) and paired non-neoplastic airway tissues, and established a biobank of 80 LCO strains of five lung cancer subtypes and five normal bronchial organoids (NBOs). It was demonstrated that the newly established LCO and NBO biobanks maintained the histological and genetic characteristics of their respective parental tissues and are expected to be used for patient-specific drug trials and proof-of-concept studies of targeted therapies and resistance mechanisms.
Results
01 LCOs established from five subtypes of cancer tissues
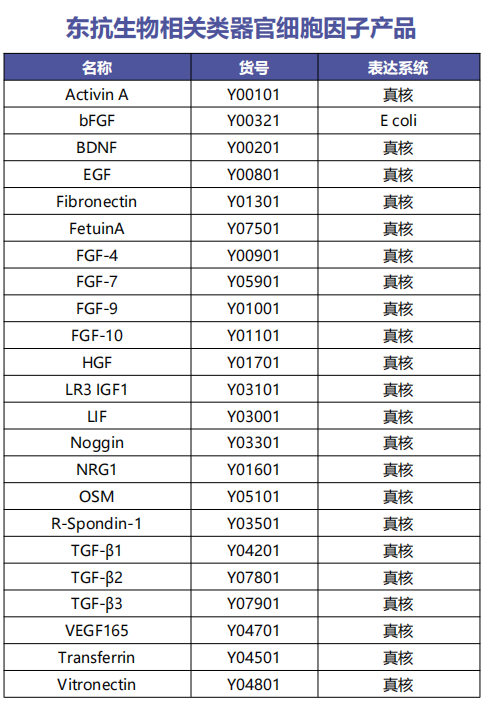
To establish LCOs from LC tissues, we developed a 3D culture protocol using minimal basal medium (MBM) in Matrigel, a suboptimal medium that inhibits normal cell growth due to depletion of Wnt3a and Noggin. MBM contains epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and N2 supplemented with insulin and transferrin. Surgically resected LC tissues were dissociated into single cells or cell clusters, embedded in Matrigel, and then immersed in MBM. Although our MBM contained fewer reagents and growth factors compared to other protocols containing multiple reagents and growth factors (including FGF-10, FGF-4, FGF-7, Noggin, and Wnt3a), LCOs extracted from LC tissues were always round, as shown in Figure 1a. As shown in Figure 1b, these LCOs expanded long-term over 6 months without any changes in spherical organoid morphology and maintained proliferation capacity by immunolabeling with the marker Ki67 (Figures a-c). In addition, we attempted to use small biopsy tissues for organoid culture to determine whether our culture protocol is applicable to samples obtained from inoperable clinical settings. As shown in Figure 1d, biopsy cells were successfully cultured to form organoids. 80 LCO lines were stored from five subtypes of lung cancer, including the 20 LCOs generated above: adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, large cell carcinoma, and small cell carcinoma (Figure 1e). One of the key factors for organoid biobanks is the efficient reconstruction of cryopreserved organoids. Therefore, we performed thawing tests on cryopreserved organoids in the biobank. Overall, 39 out of 56 LCOs (70%) successfully reconstructed the organoid morphology and histological characteristics of the original tissue in our 3D culture protocol (Figure 1f).
In summary, the LCO culture protocol was demonstrated to be an effective and reproducible method for establishing lung cancer organoid biobanks.
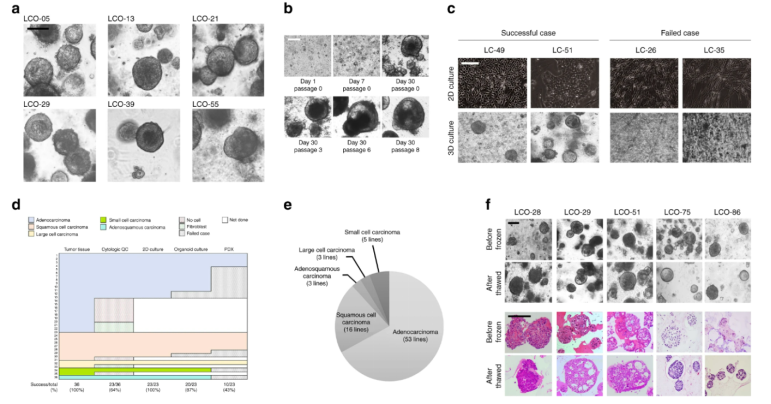
Figure 1
02 NBO maintains bronchial epithelial tissue
It is important to have normal tissue control when evaluating carcinogenesis and drug toxicity. To address this issue, non-tumor bronchial mucosa adjacent to cancer tissue was dissected and cultured using suboptimal MBM under the same culture conditions as LCO. Initially, these normal bronchial cells formed organoids; in subsequent subcultures, they failed to form organoids (Figure 2a). Induction of Wnt signaling and inhibition of BMP/TGFβ signaling promotes tissue renewal; the following lung development supplements were added to the culture medium: Noggin (BMP and TGFβ signaling blocker) and A83-01 (an activin receptor-like kinase (ALK) inhibitor) to inhibit BMP and TGFβ signaling pathways, and Wnt3a to induce Wnt signaling. Under the action of these supplements, bronchial cells successfully formed organoid tissue after 10 passages. In this case, NBO had a round shape similar to the normal lung organoids reported previously. When these organoids were grown, budding tubular organoids appeared from the 4th passage and maintained the morphology of long-term expansion in the subsequent 10 passages (Figure 2a). The data showed that these organoids had pseudostratified epithelial cells, composed of basal cells and luminal cells, including secretory cells and ciliated cells, which were the same as in normal bronchial mucosal tissue (Figure 2b). Immunofluorescence (IF) revealed that organoids consisted of basal cells expressing p63 in the outer layer of cells, and mucin-secreting cells (goblet cells) expressing MUC1 in the luminal cell layer, and ciliated cells expressing acetylated tubulin and Arl13b (Fig. 2c). In addition, some organoids also included club cells expressing CC10 (Fig. 2c). However, the distribution of cell types in NBOs was different from that in LCOs. Adenocarcinoma organoids had only MUC1-positive cells and few ciliated cells (2d), while squamous cell carcinoma organoids consisted of p63-positive cells and few ciliated cells. The types of cilia in NBOs and LCOs were different; NBOs had large bundles of motile cilia on the luminal surface of the inner layer of cells, but cells in LCOs had primary cilia with only one appendage per cell (yellow boxes in Fig. 2c-e). Here, we demonstrated that NBOs represent the spatial arrangement of normal bronchial mucosa.
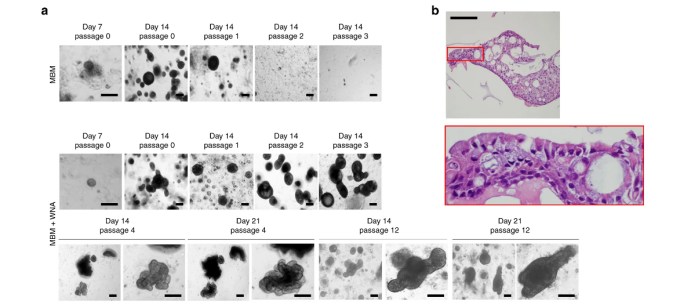
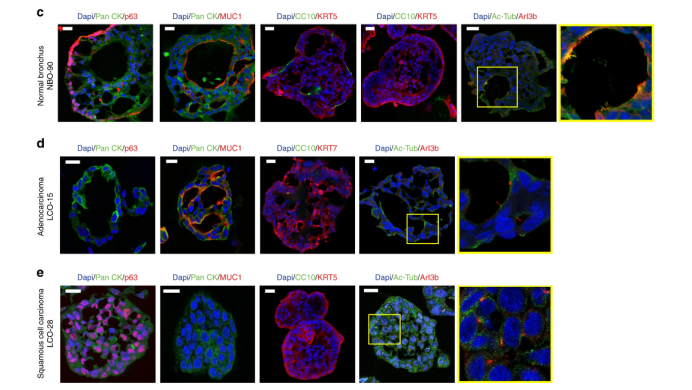
Figure 2
03 LCOs for in vitro patient-specific drug testing
To evaluate whether our LCOs can be used for in vitro drug sensitivity testing, we generated dose-response curves for several drugs and calculated the half-maximal inhibitory concentration (IC50). Docetaxel, which targets cellular microtubules 50, induced cell death in various LCOs as well as NBOs with different sensitivities. Notably, NBO are highly susceptible to the cytotoxic drug docetaxel (IC50= 0.08 μM) and the targeted drugs olaparib (IC50= 69μM), erlotinib (IC50> 100 μM) and crizotinib (IC50= 3μM) (Fig. 3a-c, f), g. Immunoblotting analysis showed changes in the expression of proteins mediating EGFR signaling and c-Met signaling after erlotinib and crizotinib treatment. Two LCOs, LCO-43 and LCO-51, were treated with 1μM erlotinib and 1μM crizotinib for 24, 48 and 72 hours, and GAPDH was used as a loading control (Fig. 3g).
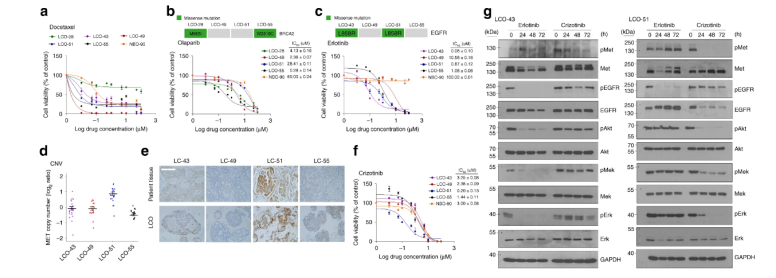
Figure 3
The results show that our LCO can be used to predict in vitro patient-specific drug responses and proof-of-concept studies of new targeted drugs based on genetic alterations.
In summary, the established LCO reproduces the histological and genetic features of the five most common lung cancer subtypes. The LCO also maintains the genomic heterogeneity of the parental lung cancer. The study shows that the LCO system will become a useful platform for drug screening and new clinical trials. In addition, NBO can also be used to evaluate the toxicity of drugs to non-cancerous cells. Considering the short time from establishment to drug testing, the model can be used to predict drug responses in specific patients as well as in more extensive preclinical studies.